Editorial Note: This article is written based on topic research and editorial review.
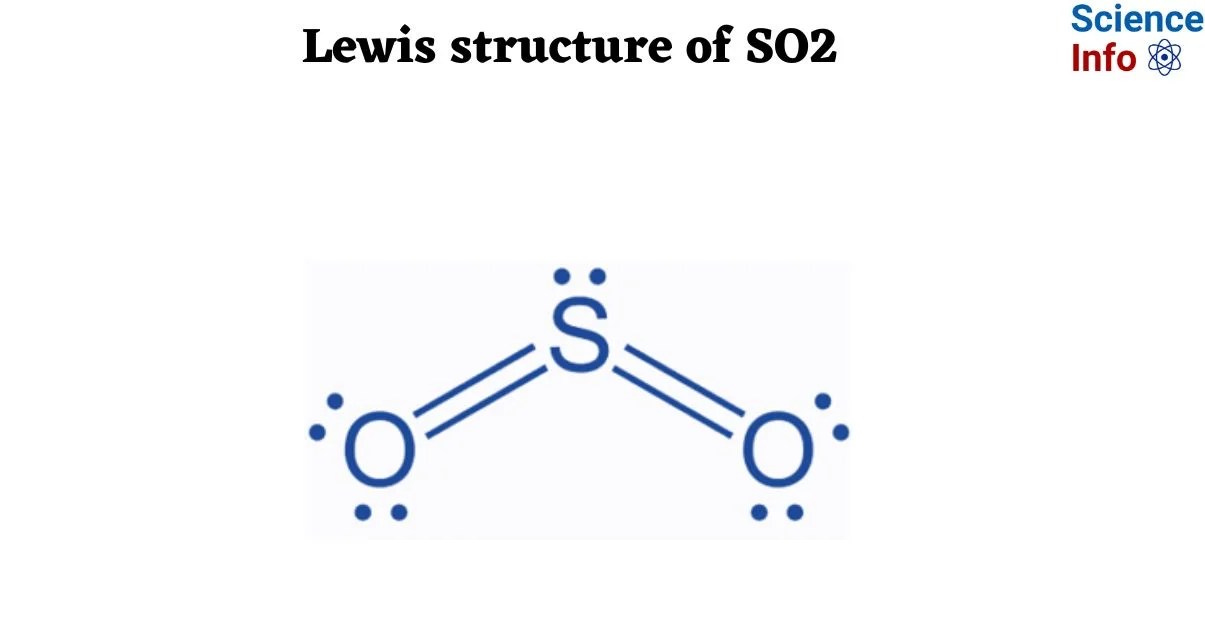
The visualization of electron distribution within a molecule, as depicted by a Lewis structure, offers fundamental insight into chemical bonding. For sulfur dioxide (SO2), this structural representation involves placing sulfur as the central atom, bonded to two oxygen atoms. The process necessitates accounting for all valence electrons, forming single and double bonds, and allocating lone pairs to achieve octets for all atoms where applicable. Crucially, SO2 exhibits resonance structures, meaning its true electron distribution is an average of multiple valid Lewis structures, contributing to its unique chemical properties and VSEPR geometry (bent molecular shape). Mastering this depiction for SO2 serves as a foundational exercise in understanding molecular architecture and electron arrangement.
Understanding Lewis structures, particularly for molecules like SO2, holds significant importance in chemistry. It provides a predictive framework for molecular geometry, polarity, and potential reactivity, which are essential for explaining a molecule's physical and chemical behavior. The ability to accurately draw and interpret these diagrams represents a critical analytical skill, fostering an innate understanding of how atoms interact to form compounds. This foundational knowledge, attributed in part to the pioneering work of Gilbert N. Lewis, is not merely an academic exercise but a practical tool that underpins advanced chemical concepts and problem-solving, cultivating an essential chemical intuition.
Proficiency in constructing and interpreting Lewis structures, exemplified by the sulfur dioxide molecule, is a pivotal step in developing a comprehensive grasp of chemical principles. This skill transcends simple diagramming; it is a gateway to predicting molecular interactions, understanding reaction mechanisms, and designing new materials. By engaging with these fundamental representations, individuals cultivate the analytical prowess necessary to navigate the complexities of molecular science, thereby empowering a deeper engagement with the subject matter and preparing for explorations into more intricate chemical phenomena.
Conclusion
The comprehensive exploration of the sulfur dioxide (SO2) Lewis structure serves as a pivotal exercise in fundamental chemical understanding. It has been demonstrated that the systematic application of principles such as valence electron summation, central atom determination, judicious electron distribution, consideration of resonance phenomena, and formal charge evaluation is indispensable. These processes collectively yield a robust representation of molecular bonding, which, for SO2, reveals its bent molecular geometry and polar nature. The ability to accurately derive and interpret such structures forms the bedrock upon which more complex chemical concepts are built, illustrating the intrinsic connection between electron arrangement and molecular properties.
The mastery attained through rigorous engagement with such foundational concepts is transformative. The precise construction and interpretation of the SO2 Lewis structure unlock an inner chemist, fostering an acute analytical capability essential for deciphering molecular complexities. This foundational skill set provides the indispensable tools for comprehensive chemical analysis, enabling a profound connection with the fundamental principles governing molecular existence and interaction. Such expertise is not merely academic; it empowers individuals to engage deeply with chemical phenomena, predict molecular behavior, and ultimately contribute to scientific inquiry and innovation.